 Loading... Please wait...
Loading... Please wait...Categories
- Home
- Help & Videos
Help & Videos
Help & Videos
Help Videos Downloads Offers
This section of our website has several how-to videos we prepared to help students become familiar with how to prepare and use their kits. We also offer examples of Lewis Structures and conformational analysis advice below.
We also have a free down-loadable version of the old Organic Chemistry Instruction booklet or if you prefer a printed copy you can buy one in our store.
We also have a free down-loadable version of the old Organic Chemistry Instruction booklet or if you prefer a printed copy you can buy one in our store.
Free Instruction Manual (Click to Download)
Helpful Information
Lewis Structures
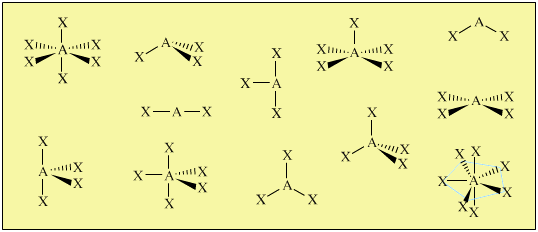
Drawing Lewis Structures can confuse students about the 3D geometry and properties of molecules and ions. Tetravalent central atoms lead to three dimensional molecules. Our models show the student the differences between electronic and molecular geometries and give valuable insights into molecular polarity. The metal clusters included are suitable for creating linear, angular, trigonal planar, tetrahedral, trigonal pyramidal, trigonal bipyramidal, See-saw, T-shaped, octahedral, square pyramidal and square planar geometries.
Co-ordination Compounds
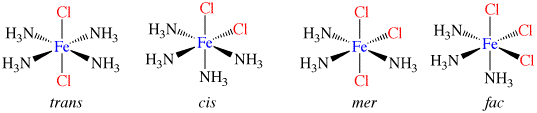
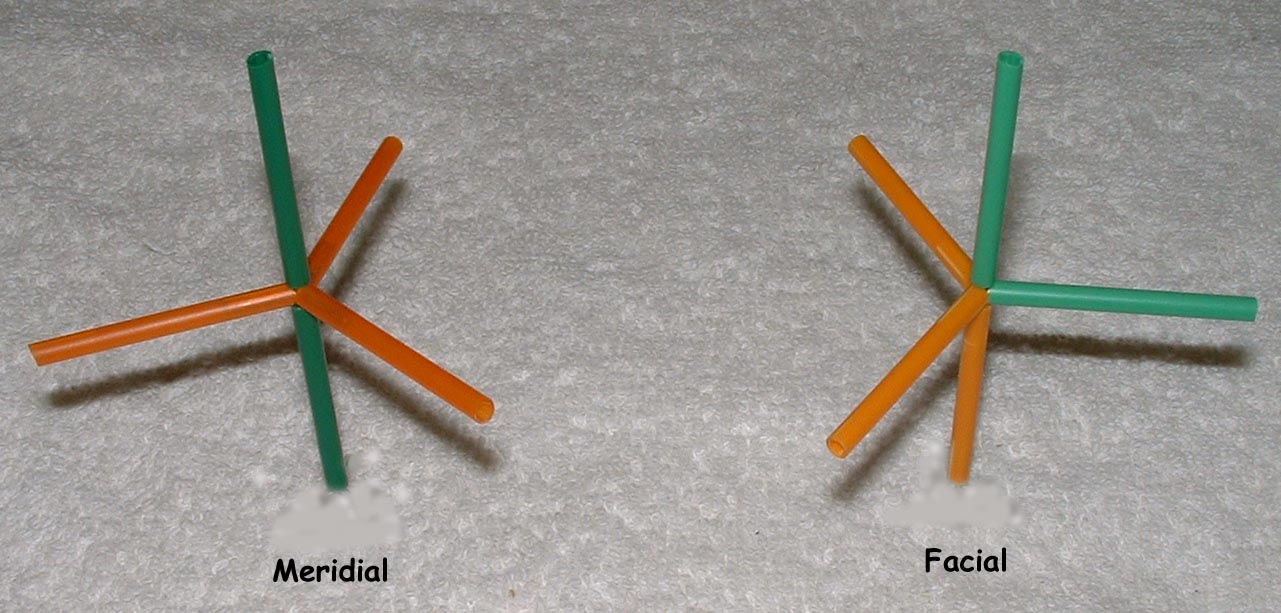
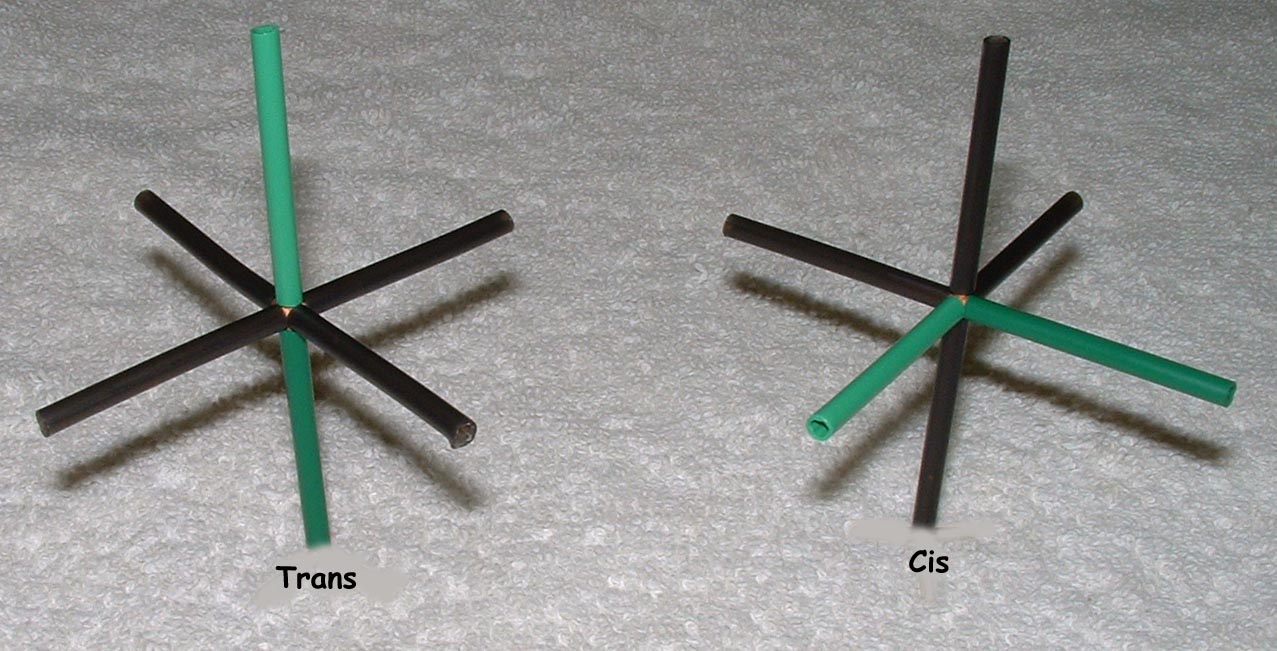
Co-ordination compounds and their expanded octet central atoms create a myriad of isomerism issues for the spatially challenged. Among them are cis/ trans, medial/facial and R and S stereo isomers that can be quickly and easily built. Enough angle pins are included to build several poly-dentate ligands at once. The metal clusters included are suitable for creating trigonal bipyramidal (5 ligand) and octahedral (6 ligand) geometries.
Conformational Analysis
The formation of carbon and heterocyclic ring structures are central to Organic Chemistry. Understanding the three dimensional structure of these rings is called conformational analysis. Determining axial and equitorial positions as the ring's chair-boat-chair inter-conversion occurs, is always difficult to visualize. 1,3-diaxial interactions become apparent using our model kits as the determinant factor in the lowest energy conformation. As students struggle to close 3 and 4 membered rings the concept of ring strain also becomes clearer.
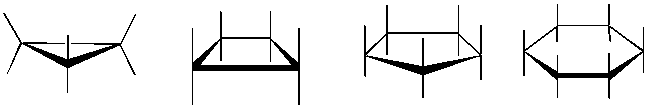

Stereoisomers
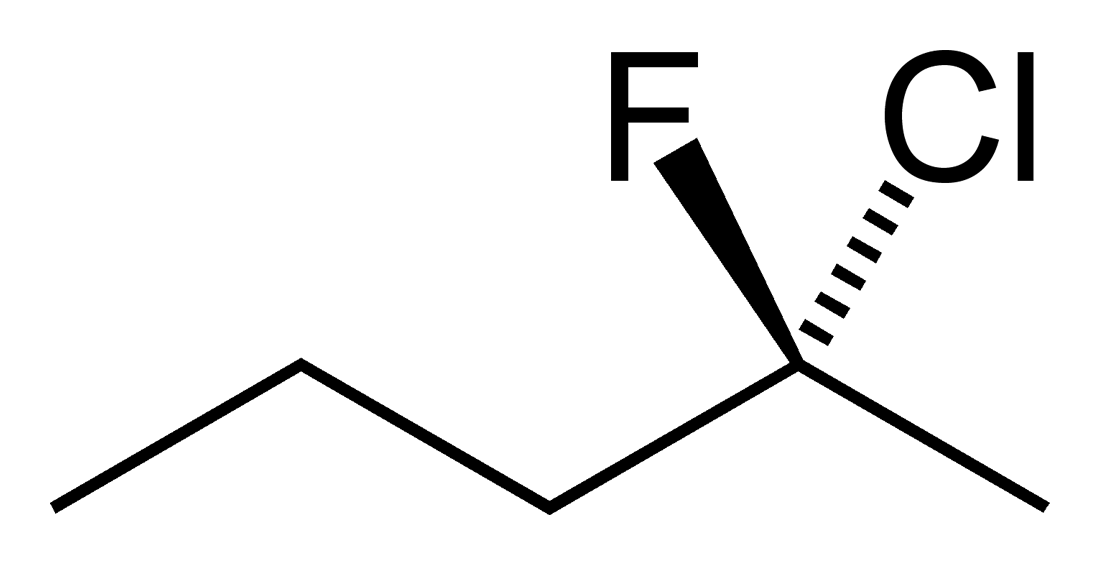
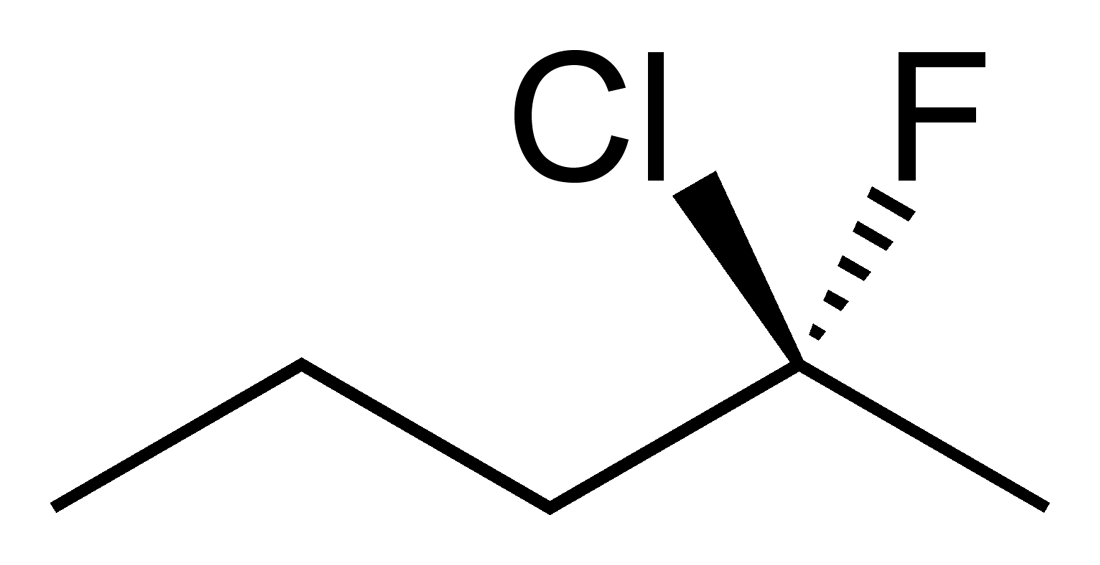
Students new to the three dimensional aspect of organic molecules can struggle with assigning R and S stereochemical designations for chiral Carbons. The ability to build the molecule and actually rotate it so that the lowest priority group is away from you can often be enlightening to these students. Determining if two molecules are enantiomers or diastereomers becomes easier with side by side comparisons. Planes of symmetry can be more easily discerned for meso compounds.
Geometric Isomers
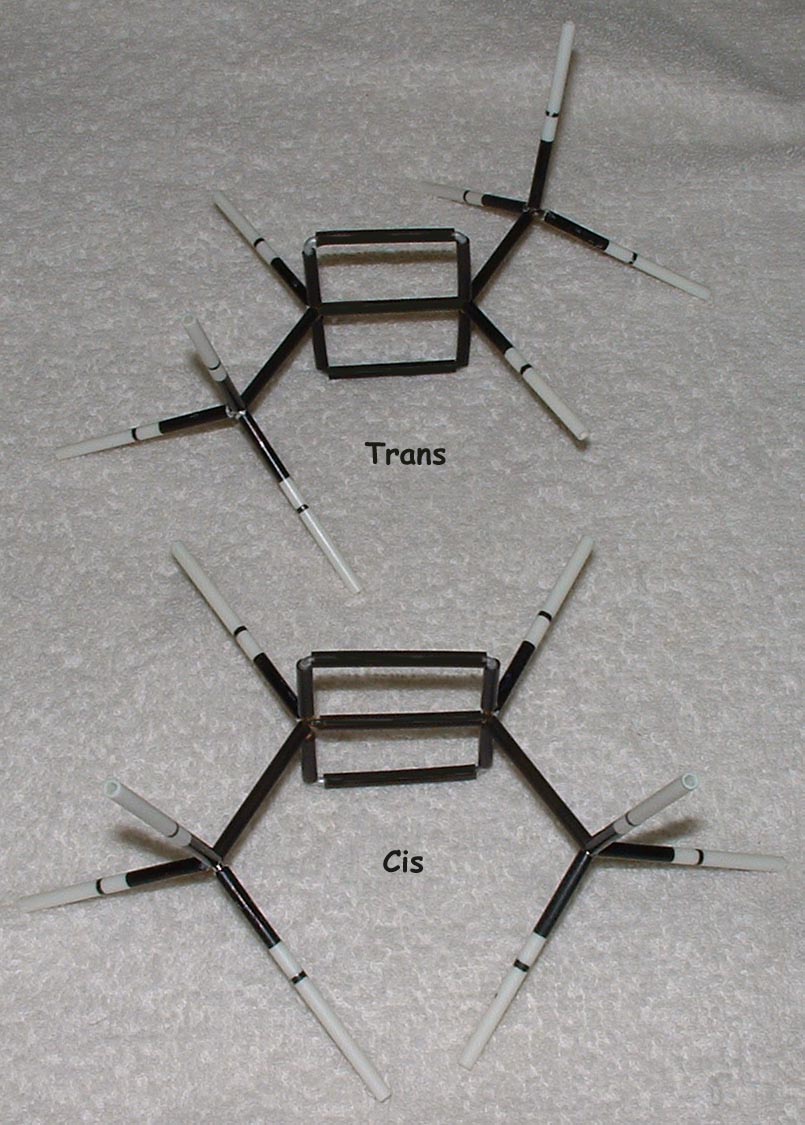
This kit is ideal for building and comparing molecules of various geometries; for example, cis and trans and E and Z alkenes. Double bonds are one of the strengths of our kits. The way that double bonds are constructed makes it obvious to students how they affect molecular geometry and how the rotation about multiple bonds is restricted.
Instructional Videos
https://youtu.be/DF52g330EF0 Intro to ChemKits kits
https://youtu.be/ODfTBHOSzkQ ChemKits Alkanes, Alkenes, Alkynes and Cyclic compounds
https://youtu.be/xBTEbjS4RzM ChemKits Cycloalkanes
https://youtu.be/us7xZnEYQJs ChemKits Cyclohexane Axial and Equatorial conformers as well as Chair Boat Inter-conversion
https://youtu.be/7wf7t-T8wyo Caffeine 2D vs 3D
https://youtu.be/8ATILaDd55k Methylcyclohexane wants to be equatorial
https://youtu.be/lRfMiNhzXIs Carbon's Allotropes
https://youtu.be/rP48FqOXR1s Using Flexible Tubing for Pi Bonds
https://youtu.be/W2sFosP02hw Building Alkenes and Alkynes with flexible tubing
https://youtu.be/DDYdEJDhnnA Building Carbonyl Pi Bonds with Flexible Tubing


